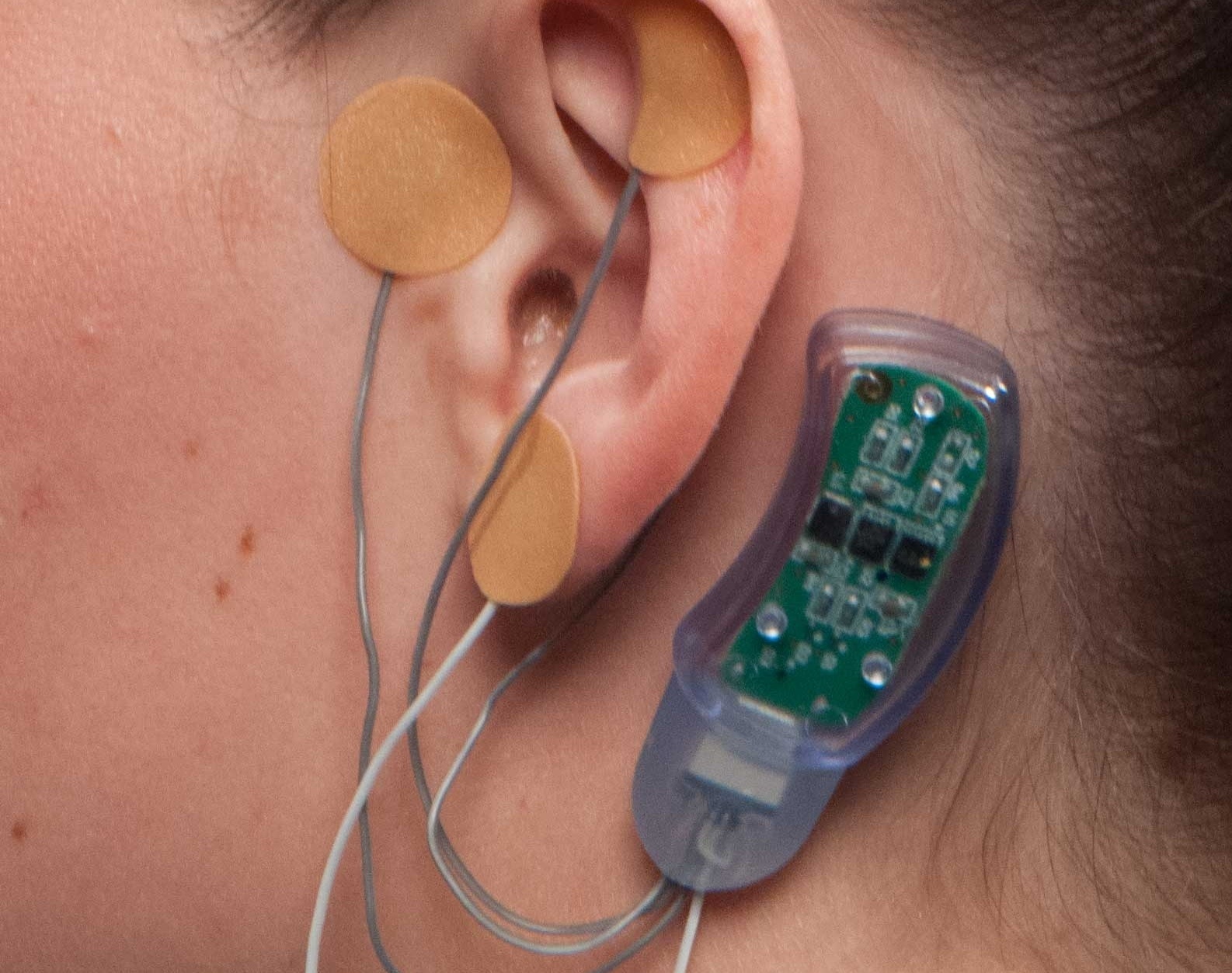
Amid an epidemic of opioid addiction, doctors are looking for ways to assist individuals to wean themselves off of the medicine, but withdrawal symptoms are a serious hurdle. Innovative Health Solutions says its NSS-2 Bridge is a “percutaneous nerve field stimulator (PNFS) device system” that sends electrical pulses to certain cranial nerves, treating symptoms together with sweating, tremors, sweating, stomach upset, joint pain and anxiety. Yesterday the FDA cleared it for marketing, making this the 1st device approved to be used in this method.